CMO Moves: Regulatory catalysts for drug manufacturing-April
Pharmaceutical Technology
JULY 14, 2022
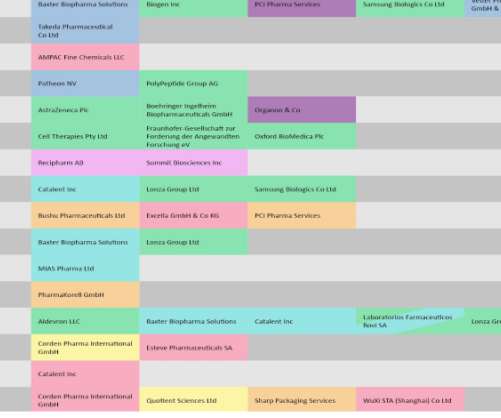
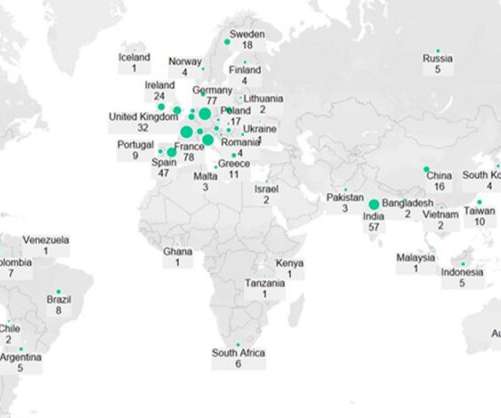
Regulatory decisions have a ripple effect, first affecting the pharma sponsors, and then the companies tasked to manufacture the drug. Pharmaceutical Technology looks at drugs and biologics with recent regulatory verdicts that will likely impact manufacturing volumes. Covid-19 vaccines stay in the spotlight.












Let's personalize your content